How did the periodic table come about?
Chemical science uses the periodic table as an invaluable tool. Simply by looking at it, we obtain a great deal of information about each of the elements. We can find out how their nucleus is formed, what their electronic configuration is like, and what their main chemical properties are.
When we look at the world around us, we perceive matter of all kinds: we can see some living bodies and others inanimate ones, some as opaque as wood and others as shiny as gold, and we touch materials as hard as a diamond or as light as air. There is no doubt that the number of substances that exist is innumerable; however, they are all made up of a combination of little more than 100 pure elements.
The question of how matter is constituted has troubled a man for a long time; as early as the 5th century B.C., the Greek philosophers Leucippus and Democritus proposed that everything that exists is made up of extremely small and indivisible bodies, which they called atoms.
This concept, which seems so logical to us today, was denied and forgotten for a long time, and it was not until the end of the 18th century and the beginning of the 19th century that it reappeared. It was the English scientist John Dalton who in 1808 established the existence of this elementary particle and defined the concept of an element as a material consisting of a single type of atom. The model proposed by Dalton for the atom has been considerably modified, however, the concept of the element is still valid.
What is the origin of the periodic table?
At the time Dalton was studying the structure of matter, he knew only about 30 pure elements, but when Joseph L. Proust formulated that the atomic masses of these elements are multiples of the masses of hydrogen, he was able to identify many others. As the number of known elements increased, the need arose to order and group them.
The first classification based on atomic properties was proposed by the German chemist J. W. Dobereiner, who, observing the behavior of many elements, grouped them into groups (three by three). Examples of these groups are those that included lithium, sodium, and potassium; calcium, strontium, and barium; and chlorine, bromine, and iodine. The elements of each triad had similar properties and Doberemer was able to establish that those of the one which, because of its weight, was located in the middle, was approximately an average of the properties of the two at the extremes.
In 1865, when 62 pure elements were already known, the English chemist J. A. R. Newlands presented another classification and organization. He had observed that when these elements were arranged in increasing order of their mass, the properties of the eighth were similar to those of the first. As this pattern was repeated every 8 elements he made an analogy with the musical scale. This last idea was not a very happy one since it was considered ridiculous by Newlands' colleagues, who did not take his proposal seriously.

Four years later, Russian scientist Dimitri Mendeleev and German scientist Lothar Meyer published almost identical schemes for classifying the elements. More credit is given to Mendeleev because he published his work a little earlier and was also more successful in demonstrating its usefulness. Like Newlands, Mendeleev realized that if the elements are arranged in ascending order of their mass, their chemical properties show a periodic repetition; he thus constructed the first periodic table of the elements, in which those with similar properties occupied the same column, with the variation of properties in the horizontal arrangement also being sequential.
The scientist found that some elements did not seem to have a place appropriate to their atomic weight, for example, argon and potassium (with atomic masses of 39.95 and 39.102 respectively) or cobalt and nickel (58.93 and 58.71). However, he decided to place them in the right place according to their properties, because he considered that this was the most important characteristic to take into account. Mendeleev had to leave empty spaces for elements not yet covered. From the properties of the known elements he deduced those of three others that were not known; and later, when scandium, gallium, and germanium were discovered, their properties turned out to be very similar to those predicted. In 1871 Mendeleev revised his table and classified the elements into 8 groups corresponding to the vertical columns.
In 1895 Alfred Werner separated the elements of groups A and B, thus presenting the long table that is used today in which the placement of the elements is related to their electronic configuration, even though it was made many years before this was known. Already in this table, the lanthanide and actinide series had only one box for each.
It was the English chemist Henry. G.J Moseley realized that chemical properties do not depend on the mass of the atom but its atomic number, a concept he developed after observing that when metals are bombarded with cathode rays the frequency of the rays emitted varies depending on the type of metal. He concluded that in the atom there is a fundamental quantity Z, which increases by regular steps when passing from one element to the next and which can only be the charge of the positive central nucleus; he indicated that Z is equal to the number of the place of the element in the periodic table.
As we can see, the periodic table that we use today derives from the works of Mendeleev, Werner, and Moseley; in it, the elements are ordered according to their increasing atomic numbers and is governed by the Moseley law that says: the properties of the elements are a periodic function of their atomic numbers.

How is the periodic table organized?
The tables of the elements
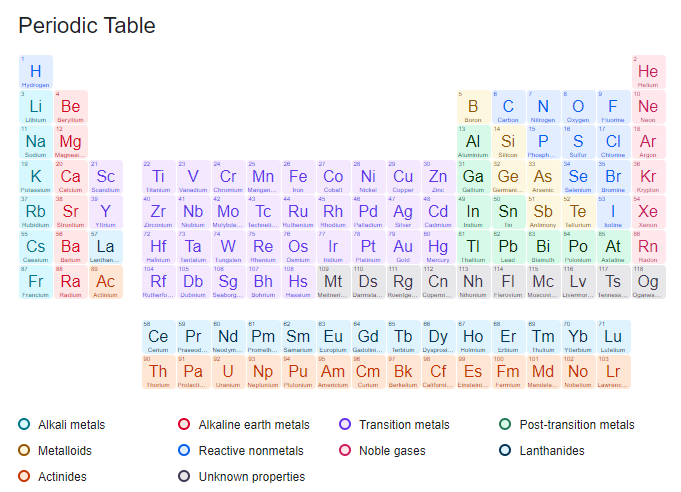
In the periodic table currently in use, we see that each element has a table. The information contained in it can be more or less complete depending on the purpose of the table, but it always includes the symbol of the element and its complete name, the atomic number, and the mass number. Some tables add other data such as the electronic configuration of all the occupied levels, the valences with which the element acts and the electronegativity value, the state of aggregation of the element at room temperature, the crystalline structure, and the acid-base properties, etc., can also be indicated.
The position in which this data is distributed may be different from table to table, so in most of them, a separate table is placed in which all the references are indicated. Currently, 109 elements are recognized, so the table has the same number of boxes.
Groups and periods
Horizontal arrangements are called periods and vertical arrangements are called groups. The table consists of seven periods that indicate how many energy levels are occupied by electrons in each element. The name of the period is indicated by Arabic numerals, which generally appear on the left-hand side of each period.
Perhaps looking at most of the tables you may think that in the 6th period there are only 18 elements, but it is very important not to forget that the lanthanide series belongs to this period, just as the actinide series belongs to the 7th period. When the inner transition metals (lanthanides and actinides) are inserted in their places, the table becomes extremely wide.
The elements of the table are grouped in 18 vertical columns which are called groups. Chemists have named them in a variety of ways, but the most common is the one explained below.
The groups are divided into A and B, the A groups are those that include the elements that have their differential electron (the last of the configuration) in the last level in which there are electrons, in the s or p sublevels; while the B groups are those elements that do not have their differential electron in the last occupied level. A number, usually Roman numerals, has been assigned which, together with the letter A or B, forms the name of the group and generally appears at the top of each group. In groups A, the group number indicates the number of electrons the atom of the element has in its last energy level.
In 1985 the Union of Pure and Applied Chemistry accepted that the groups be named with an Arabic numeral from 1 to 18, without distinguishing whether it is A or B.
Blocks
An element is placed in the table depending on its atomic number and the type of sublevel in which its electron differential is placed. Currently, only four sublevels (s, p, d, and f) are known, and therefore the table is divided into four blocks.
The elements that have their differential electron in s and p are called representative and all the A groups are in them (remember that their differential electron is in the last level occupied by electrons). The elements that have their differential electron in some orbital of the d sublevel are called transition elements and belong to the B groups, and those that have their differential electron in an f orbital are called internal transition elements.
Classes
The elements have been grouped into classes based on their metallic or nonmetallic properties. Most of the elements are metallic, only a quarter can be classified as nonmetals. Metals have a lustrous and shiny appearance, are good conductors of heat and electricity, and are malleable and ductile. Most metals are solid at room temperature, except mercury, cesium (their melting temperature is 28.7°C), and gallium (melting temperature 29-8°C). Non-metals, on the other hand, do not have much luster or shine, are opaque, and are neither malleable nor ductile. Many nonmetals are gaseous and others are solids, only bromine is liquid.
The elements on the left-hand side of the table, except for hydrogen, are metals; the nonmetals are on the right-hand side. Elements that lie on the borderline between the two and have properties intermediate between metals and nonmetals are usually called semimetals.
The Union of Pure and Applied Chemistry currently recommends grouping the elements into eight classes. These are the alkali metals, the alkaline earth metals, the transition metals, the other metals, the nonmetals, the lanthanides, the actinides, and the noble gases. The simplest element, hydrogen, is the most difficult to classify and does not belong to any class; by its electronic configuration it should be with the alkali metals, but its properties have absolutely nothing to do with them since it behaves like a nonmetal and is gaseous.
Conclusions
A periodic table is an invaluable tool in chemical science. Just by looking at it, we obtain a great deal of information about each of the elements. We can know how their nucleus is formed, what their electronic configuration is like and what their main chemical properties are. Without it, the study of matter and its elements would be very chaotic and difficult.
Author: María Jesús Arbiza Díaz




